Clinical Reviews:針對(duì)食物過(guò)敏的表皮免疫療法(綜述)
發(fā)布日期:2018-11-13
原標(biāo)題:食物過(guò)敏表皮免疫治療現(xiàn)狀
延伸閱讀

Clinical Reviews in Allergy & Immunology
DOI: org/10.1007/s12016-017-8650-3
Abstract:
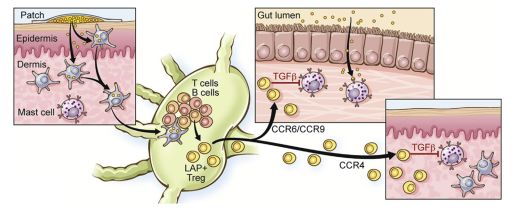
The food allergy epidemic of recent years has led to the search for safe and effective methods of immunotherapy for foods. Studies of epicutaneous immunotherapy (EPIT) in mice have shown promising safety and efficacy data. Murine models have also identified probable mechanisms for the development of tolerance to food allergens, including the induction of regulatory T cells. Clinical data is lacking, but relatively small and early studies among peanut and cow’s milk allergic subjects suggest that EPIT has an excellent safety profile,particularly compared to other methods of specific allergen immunotherapy. Efficacy data are also promising for peanut allergy, among younger patients (ages 4–11 years of age),suggesting that a majority of young patients will experience an increase in reaction threshold with therapy. The goal of this therapy is the protection from accidental exposures to a known food allergen. Additional clinical data is needed to prove efficacy and further demonstrate the safety profile of EPIT for food allergy, prior to approval by the Food and Drug Administration.
First Author:
Bruce J. Lanser
Correspondence:
National Jewish Health
All Authors:
Bruce J. Lanser, Donald Y. M. Leung


——來(lái)自浙大迪迅
① 近年來(lái)食物過(guò)敏的流行導(dǎo)致急需尋找安全有效的免疫治療方式;② 皮膚免疫治療(EPIT)在小鼠中的研究得到了安全有效的數(shù)據(jù);③ 小鼠模型還發(fā)現(xiàn)了一些可能對(duì)食物過(guò)敏原產(chǎn)生耐受性機(jī)制,包括調(diào)節(jié)性T細(xì)胞的誘導(dǎo);④ 該方面研究目前缺乏臨床資料,但在一些小型的早期研究表明,對(duì)于花生和牛奶的過(guò)敏患者,EPIT比其他特異性變應(yīng)原免疫治療方法更具有安全性;⑤ 該療法的目的是防止意外暴露于已知的食物過(guò)敏原。
延伸閱讀

Clinical Reviews in Allergy & Immunology
[IF:6.442]
The Current State of Epicutaneous Immunotherapy for Food Allergy: a Comprehensive ReviewDOI: org/10.1007/s12016-017-8650-3
Abstract:
The food allergy epidemic of recent years has led to the search for safe and effective methods of immunotherapy for foods. Studies of epicutaneous immunotherapy (EPIT) in mice have shown promising safety and efficacy data. Murine models have also identified probable mechanisms for the development of tolerance to food allergens, including the induction of regulatory T cells. Clinical data is lacking, but relatively small and early studies among peanut and cow’s milk allergic subjects suggest that EPIT has an excellent safety profile,particularly compared to other methods of specific allergen immunotherapy. Efficacy data are also promising for peanut allergy, among younger patients (ages 4–11 years of age),suggesting that a majority of young patients will experience an increase in reaction threshold with therapy. The goal of this therapy is the protection from accidental exposures to a known food allergen. Additional clinical data is needed to prove efficacy and further demonstrate the safety profile of EPIT for food allergy, prior to approval by the Food and Drug Administration.
First Author:
Bruce J. Lanser
Correspondence:
National Jewish Health
All Authors:
Bruce J. Lanser, Donald Y. M. Leung
2018-10-25 Review
創(chuàng)建過(guò)敏性疾病的科研、科普知識(shí)交流平臺(tái),為過(guò)敏患者提供專業(yè)診斷、治療、預(yù)防的共享平臺(tái)。

 杭州浙大迪迅生物基因工程有限公司
杭州浙大迪迅生物基因工程有限公司

