JACI:用Alt a 1進(jìn)行變應(yīng)原特異性免疫治療的對照研究
發(fā)布日期:2019-05-17
原標(biāo)題:用主要變應(yīng)原Alt a 1進(jìn)行變應(yīng)原特異性免疫治療的雙盲、隨機(jī)、安慰劑對照試驗(yàn)
延伸閱讀
JACI [IF:13.1]
Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1
https://doi.org/10.1016/j.jaci.2019.02.029
Abstract:
Background
There have been few studies conducted on the efficacy and safety of specific immunotherapy with allergen extracts of fungi compared with other allergen extracts, and there are no data on the major allergen Alt a 1 of the fungus Alternaria alternata.
Objectives
We sought to evaluate the efficacy and safety of subcutaneous immunotherapy with 2 different doses of Alt a 1 in patients with rhinoconjunctivitis caused by sensitization to A alternata.
Method
We performed a multicenter, randomized, double-blind, placebo-controlled trial with Alt a 1 administered subcutaneously in patients with allergic rhinoconjunctivitis with or without controlled asthma aged 12 to 65 years. Three groups were included: the placebo group and active groups receiving 0.2 or 0.37 μg of Alt a 1 per dose. The main end point was the combined symptom and medication score. Secondary end points were cutaneous reactivity and serum IgE and IgG4 levels to Alt a 1. Recorded adverse reactions were graded according to World Allergy Organization criteria.
Results
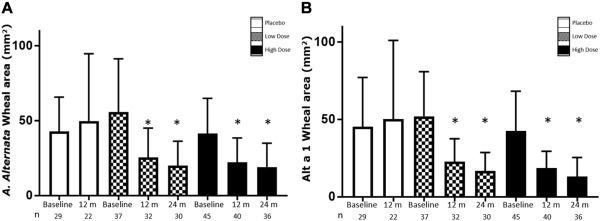
There were significant reductions in the combined symptom and medication score for the 0.37-μg dose of Alt a 1 compared with placebo at 12 months of treatment. Reduced cutaneous reactivity and IgE levels, together with increased IgG4 levels, were demonstrated for the 2 active groups versus the placebo group. A similar safety profile was found for both active groups compared with the placebo group. No serious adverse drug reactions were reported.
Conclusion
Immunotherapy with Alt a 1 was efficacious and safe, reducing the symptoms and medication consumption associated with rhinoconjunctivitis after only 1 year of treatment. The clinical benefits were associated with reduced skin reactivity and specific IgE levels and increased IgG4 levels.
All Author:
Ana Isabel Tabar Luis Prieto Pilar Alba Antonio Nieto Miguel Torrecillas
Mercedes Rodríguez Beatriz Huertas Elisa Gómez Francisco Javier Fernández Ricardo Palacios Miguel Blanca David Rodríguez
xMartin Lloyd HibberdSearch for articles by this authorAffiliations
· Genome Institute of Singapore, Agency for Science, Technology and Research Singapore, Singapore


——浙大迪迅 譯
?、倥c用其他變應(yīng)原提取物進(jìn)行特異性免疫治療相比,用真菌變應(yīng)原提取物進(jìn)行免疫治療的有效性和安全性研究較少,對交鏈孢霉主要變應(yīng)原Alt a1的研究也未見報(bào)道。②我們試圖評估2種不同劑量的Alt a1皮下免疫治療由鏈格孢引起的鼻結(jié)膜炎的療效和安全性。③我們進(jìn)行了一項(xiàng)多中心、隨機(jī)、雙盲、安慰劑對照試驗(yàn),對年齡在12至65歲之間的過敏性鼻結(jié)膜炎合并或不合并哮喘的患者進(jìn)行皮下注射Alt a1。三組包括:安慰劑組和每劑接受0.2或0.37μg Alt 1的兩組治療組治療。主要終點(diǎn)為癥狀與用藥評分的結(jié)合。次要終點(diǎn)為皮膚反應(yīng)性、針對Alt a1的血清IgE和IgG4水平的變化。不良反應(yīng)的記錄按照世界過敏組織的標(biāo)準(zhǔn)進(jìn)行分級。④與安慰劑相比,在12個(gè)月的治療后,0.37 -μg劑量的Alt 1治療組的癥狀和藥物結(jié)合評分有顯著減少。與安慰劑組相比,兩組患者的皮膚反應(yīng)性和IgE水平降低,IgG4水平升高。與安慰劑組相比,兩治療組的安全性相似。無嚴(yán)重藥物不良反應(yīng)報(bào)告。⑤ Alt a1免疫治療是有效和安全的,僅經(jīng)過1年的治療,鼻結(jié)膜炎的癥狀和藥物需要量均減少。臨床療效與皮膚反應(yīng)性和特異性IgE水平的降低以及特異性IgG4水平的增加有關(guān)。
延伸閱讀
JACI [IF:13.1]
Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1
https://doi.org/10.1016/j.jaci.2019.02.029
Abstract:
Background
There have been few studies conducted on the efficacy and safety of specific immunotherapy with allergen extracts of fungi compared with other allergen extracts, and there are no data on the major allergen Alt a 1 of the fungus Alternaria alternata.
Objectives
We sought to evaluate the efficacy and safety of subcutaneous immunotherapy with 2 different doses of Alt a 1 in patients with rhinoconjunctivitis caused by sensitization to A alternata.
Method
We performed a multicenter, randomized, double-blind, placebo-controlled trial with Alt a 1 administered subcutaneously in patients with allergic rhinoconjunctivitis with or without controlled asthma aged 12 to 65 years. Three groups were included: the placebo group and active groups receiving 0.2 or 0.37 μg of Alt a 1 per dose. The main end point was the combined symptom and medication score. Secondary end points were cutaneous reactivity and serum IgE and IgG4 levels to Alt a 1. Recorded adverse reactions were graded according to World Allergy Organization criteria.
Results
There were significant reductions in the combined symptom and medication score for the 0.37-μg dose of Alt a 1 compared with placebo at 12 months of treatment. Reduced cutaneous reactivity and IgE levels, together with increased IgG4 levels, were demonstrated for the 2 active groups versus the placebo group. A similar safety profile was found for both active groups compared with the placebo group. No serious adverse drug reactions were reported.
Conclusion
Immunotherapy with Alt a 1 was efficacious and safe, reducing the symptoms and medication consumption associated with rhinoconjunctivitis after only 1 year of treatment. The clinical benefits were associated with reduced skin reactivity and specific IgE levels and increased IgG4 levels.
All Author:
Ana Isabel Tabar Luis Prieto Pilar Alba Antonio Nieto Miguel Torrecillas
Mercedes Rodríguez Beatriz Huertas Elisa Gómez Francisco Javier Fernández Ricardo Palacios Miguel Blanca David Rodríguez
xMartin Lloyd HibberdSearch for articles by this authorAffiliations
· Genome Institute of Singapore, Agency for Science, Technology and Research Singapore, Singapore
2019-5-2 Artical
創(chuàng)建過敏性疾病的科研、科普知識交流平臺,為過敏患者提供專業(yè)診斷、治療、預(yù)防的共享平臺。

 杭州浙大迪迅生物基因工程有限公司
杭州浙大迪迅生物基因工程有限公司

