JACI:蜜蜂毒液過敏原組份診斷的新發(fā)現(xiàn)
發(fā)布日期:2018-12-18
原標(biāo)題:蜜蜂毒液過敏病人中應(yīng)用過敏原組份診斷發(fā)現(xiàn)新的主要過敏原


——來自浙大迪迅
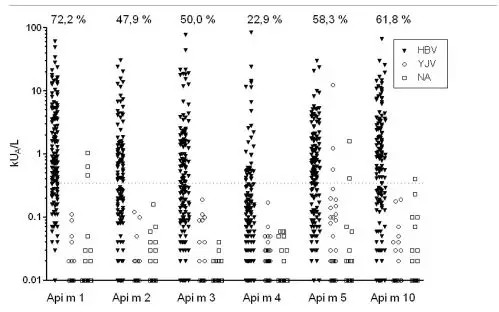
?、僦亟M膜翅目毒液過敏原的IgE檢測(cè)被認(rèn)為可以提高膜翅目毒液過敏的診斷正確性。然而,對(duì)蜜蜂毒液過敏患者唯一可用的重組蜂毒(HBV)過敏原rApi m1的致敏頻率是有限的,這表明可能存在另外的蜜蜂毒液過敏原。②我們對(duì)蜜蜂毒液過敏病人進(jìn)行一系列蜜蜂毒液過敏原(組份)的敏感性分析。蜜蜂毒液過敏(HBV)的診斷(n = 144)是基于病史、皮膚試驗(yàn)結(jié)果和對(duì)HBV的過敏原特異性IgE水平。采用ImmunoCAP分析IgE對(duì)6種無交叉反應(yīng)碳水化合物(CCD)的HBV過敏原的反應(yīng)性。④.IgE對(duì)rApi m1、rApi m2、rApi m3、nApi m4、rApi m5、rApi m10的反應(yīng)頻率分別為72.2%、47.9%、50.0%、22.9%、58.3%、61.8%。94.4%的患者對(duì)至少1種HBV過敏原呈陽性反應(yīng)。在68.0%的患者中檢測(cè)到IgE對(duì)Api m3、Api m10或兩者都有反應(yīng),并在5%的患者中是唯一的HBV過敏原特異性IgE。治療性HBV對(duì)IgE結(jié)合的抑制有限,在獲得免疫治療的患者中對(duì)Api m3和Api m 10特異性IgG4的誘導(dǎo)有限,這些都支持了最近關(guān)于這些過敏原在治療性HBV制劑中表達(dá)不足的報(bào)道。⑤與單獨(dú)使用rApi m1相比,對(duì)一組無CCD(碳水化合物交叉反應(yīng)決定簇)的HBV過敏原(組份)的分析提高了診斷敏感性,識(shí)別了其它主要過敏原,并揭示了在治療性HBV制劑中已報(bào)道的缺失或未充分表達(dá)的過敏原(組份)的敏感性。
延伸閱讀
JACI:
[IF:13.1]
Component resolution reveals additional major allergens in patients with honeybee venom allergy
https://doi.org/10.1016/j.jaci.2013.10.060
Abstract:
Background
Detection of IgE to recombinant Hymenoptera venom allergens has been suggested to improve the diagnostic precision in Hymenoptera venom allergy. However, the frequency of sensitization to the only available recombinant honeybee venom (HBV) allergen, rApi m 1, in patients with HBV allergy is limited, suggesting that additional HBV allergens might be of relevance.
Objective
We performed an analysis of sensitization profiles of patients with HBV allergy to a panel of HBV allergens.
Methods
Diagnosis of HBV allergy (n = 144) was based on history, skin test results, and allergen-specific IgE levels to HBV. IgE reactivity to 6 HBV allergens devoid of cross-reactive carbohydrate determinants (CCD) was analyzed by ImmunoCAP.
Results
IgE reactivity to rApi m 1, rApi m 2, rApi m 3, nApi m 4, rApi m 5, and rApi m 10 was detected in 72.2%, 47.9%, 50.0%, 22.9%, 58.3%, and 61.8% of the patients with HBV allergy, respectively. Positive results to at least 1 HBV allergen were detected in 94.4%. IgE reactivity to Api m 3, Api m 10, or both was detected in 68.0% and represented the only HBV allergen–specific IgE in 5% of the patients. Limited inhibition of IgE binding by therapeutic HBV and limited induction of Api m 3– and Api m 10–specific IgG4 in patients obtaining immunotherapy supports recent reports on the underrepresentation of these al lergens in therapeutic HBV preparations
Conclusion
Analysis of a panel of CCD-free HBV allergens improved diagnostic sensitivity compared with use of rApi m 1 alone, identified additional major allergens, and revealed sensitizations to allergens that have been reported to be absent or underrepresented in therapeutic HBV preparations.
All Author:
JulianK?hlerMSa?SimonBlankPhDb?SabineMüllerMDaFrankBantleonDiplBiolbMarcelFrickMSaJohannesHuss-MarpMDacJonasLidholmPhDdEdzardSpillnerPhDb?ThiloJakobMDa?
2018-12-10 Article
創(chuàng)建過敏性疾病的科研、科普知識(shí)交流平臺(tái),為過敏患者提供專業(yè)診斷、治療、預(yù)防的共享平臺(tái)。

 杭州浙大迪迅生物基因工程有限公司
杭州浙大迪迅生物基因工程有限公司

