Release date:2019-02-20
JACI
[IF:13.1]
Diagnosis of dog allergy: Beware of the dog
October 2018Volume 142, Issue 4, Pages 1058–1059
DOI:DOI: https://doi.org/10.1016/j.jaci.2018.08.006
Exposure to furred pets is high. In Europe dog ownership varies from 9.0% to 34.8%,1
whereas in the United States 30.4% of the households own cats and 36.5% own dogs.2
However, the diagnosis of dog allergy is troublesome. In general, self-reporting misclassifies allergic status in many patients. Even a structured allergy history alone is little better and results in false-positive rates for dog allergy of 27% compared with formal allergy assessment.
In a more recent study, it was found that skin prick test response positivity to dog was associated with a history of symptoms on exposure to dog.4
Although significant, the magnitude of the association was not high. For a positive predictive value of 80%, the wheal diameter cutoff amounted to 10 mm.
In addition, the diagnosis of dog allergy is hampered by varying levels of allergen content when comparing skin prick test extracts from different sources.5
In this study 5 extracts from European manufacturers were examined. Skin prick test extracts showed a 20-fold variation in protein content, whereas the dog allergens Can f 1 and Can f 2 varied widely between extracts, with undetectable levels in 1 of 5 extracts.
Identification of the distinct dog allergens Can f 1, Can f 2, Can f 4, and Can f 6 (belonging to the lipocalin family); the dog serum albumin Can f 3; and the prostatic kallikrein Can f 5 have improved the diagnostic approach for patients sensitized to dog.6
The relevance of the recently identified Can f 7 (dog epididymal secretory protein) is not known. Can f 1 is a major dog allergen recognized by 50% to 90% of patients sensitized to dog. The lipocalins Can f 2 and Can f 6 and the serum albumin Can f 3 are cross-reactive to other furred animals. Monosensitization to Can f 5 has been suggested as a marker for specific allergy to male dogs. Thus with this set of components, it is possible to discriminate between primary sensitization to dog, primary sensitization to dog and potentially cross-reactivity or cosensitization to another animal, or primary sensitization to cat or horse with cross-sensitization to dog.6
It is not always clear whether sensitization to dog allergen components points to clinical relevance. By using double-blind, placebo-controlled food challenges, it has been shown that IgE to peanut components, such as Ara h 2 and Ara h 6, can be associated with systemic reactions in children sensitized to peanut. Such studies have not been done with inhalant allergens.
aimed to study the association between sensitization and clinical symptoms of dog allergy as assessed by using nasal challenge testing in 60 children (age 10-18 years) sensitized to dog. They found that a positive nasal provocation test result was associated with sensitization to an increasing number of dog allergen components. Sensitization to lipocalins, in particular to Can f 4 and Can f 6, was related to a positive challenge result. The presence of IgE to Can f 1 was not associated with a positive provocation test result, possibly because the majority of children were sensitized to Can f 1. Nevertheless, the clinical relevance of Can f 1 was illustrated by a strong relationship between the increasing level of IgE to Can f 1 and a positive outcome on the challenge test. Can f 3 was the least common sensitizing component and not related to a positive test result. Because IgE to Can f 3 was mostly seen in multisensitized patients, IgE to dog serum albumin is perhaps a better marker for cross-reactivity than for clinically relevant allergy. Analysis of the relation between sensitization profiles showed that the highest odds ratio for a positive nasal provocation test result was found among patients who were sensitized to all protein groups (ie, lipocalins, serum albumin, and prostatic kallikrein [odds ratio, 5.34; 95% CI, 1.01-28.4]), whereas monosensitization to Can f 5 was associated with a negative challenge result.
used the nasal challenge test as the gold standard for assessing clinical allergy to dogs. Nasal provocation tests are not regularly used in daily practice, but they are a valuable tool in research, particularly in clinical trials.
A limitation of this study is that nasal challenge procedures are not well standardized in general. There are a variety of methods to assess nasal reactivity to allergens using symptom scores and/or objective measurements of nasal patency, such as rhinomanometry, acoustic rhinometry, or peak nasal inspiratory flow.8
However, the method used by K?ck et al7
has been used in different types of studies and is a well-accepted tool included in a recently published European Academy of Allergy and Clinical Immunology position paper on nasal provocation tests.8
Another drawback of the nasal challenge test is that the limitations of skin testing (ie, variability in allergen content) might also be true for allergenic extracts used for challenge. The allergen solution used in this study was well characterized, with all relevant dog components measured and present in the extract. Finally, taking the inaccuracy of the history into account,3
the challenge test might be the best available reference for evaluating sensitization patterns to different dog allergens.
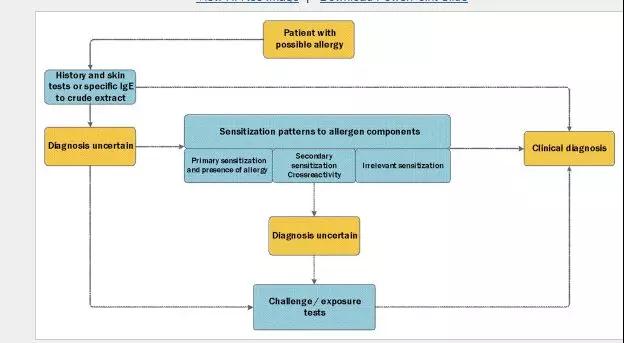
The major challenge in managing allergic patients is always to differentiate between irrelevant sensitization and clinical allergy. If the combination of a history with conventional skin prick tests or specific IgE to the crude extract is insufficient to establish the diagnosis, a good alternative is use of exposure tests (nasal and conjunctival provocation tests or oral food challenges). Although less laborious and less burdensome for patients than food challenge tests, nasal and conjunctival provocations are not commonly used in daily practice. Apart from standardization issues, lack of allergenic extracts for challenge tests that are registered by national regulators might hamper use of these tests. The availability of allergen components has provided the clinician with tools to better identify clinically relevant sensitizations. This is particularly true for food allergy in children, but the study by K?ck et el7 shows that this can also be the case in the setting of inhalant allergy. The effect of analyzing IgE patterns to allergen components might vary per allergen, but an increasing number of studies underwrite the added value of determining levels of IgE to specific allergens in the diagnostic process. Although the study by K?ck et al7 does not point to a single marker to detect clinical relevance, determination of the pattern of IgE sensitization to dog components might be helpful in recognizing true allergy. The approach of comparing sensitization to specific components with nasal or conjunctival challenge tests could also be valuable to identify the relevant allergens for patients with other inhalant allergies. Depending on the suspected allergens, analysis of IgE patterns to allergen components may be helpful in the diagnosis of allergy (Fig 1).
Author:
MD, PhD Roy Gerth van Wijk
2019-2-15 Artical
 杭州浙大迪迅生物基因工程有限公司
杭州浙大迪迅生物基因工程有限公司
